Where does lead come from?
Lead is most commonly found as lead (II) sulphide (PbS) in the mineral galenite. Cerussite (tin-lead ore), with the chemical composition lead (II) carbonate (PbCO₃), is a secondary mineral of lead formed by the chemical reaction of carbonated water with galena. A third important source of lead is anglesite (lead vitriol), which is lead (II) sulphate (PbSO₄), formed by the oxidation of galena.
|
Galenite |
Anglesite |
Cerussite |
 |
 |
 |
| Table 1: Lead minerals | |
| Mineral | Chemical formula |
| Galenite | Lead (II) sulphide (PbS) |
| Anglesite | Lead (II) sulphate (PbSO₄) |
| Cerussite | Lead (II) carbonate (PbCO₃) |
Is lead still used?
Lead is used in many different applications including:
- Lead-acid storage batteries
- Rolled lead sheets which are used in the building industry for the water proofing
of roofing - Lead bullets in ammunition
- Paint pigment
- Anti-fouling component in coating systems
How is lead present in groundwater?
The Pourbaix or potential/pH diagram in Figure 1 of lead shows the lead speciation in water. The area between the orange dotted lines represents the stability zone of water. The diagram shows that the lead speciation in water is dominated by its cationic elementary Pb²⁺form.
Figure 1: This is the Pourbaix diagram of lead in water
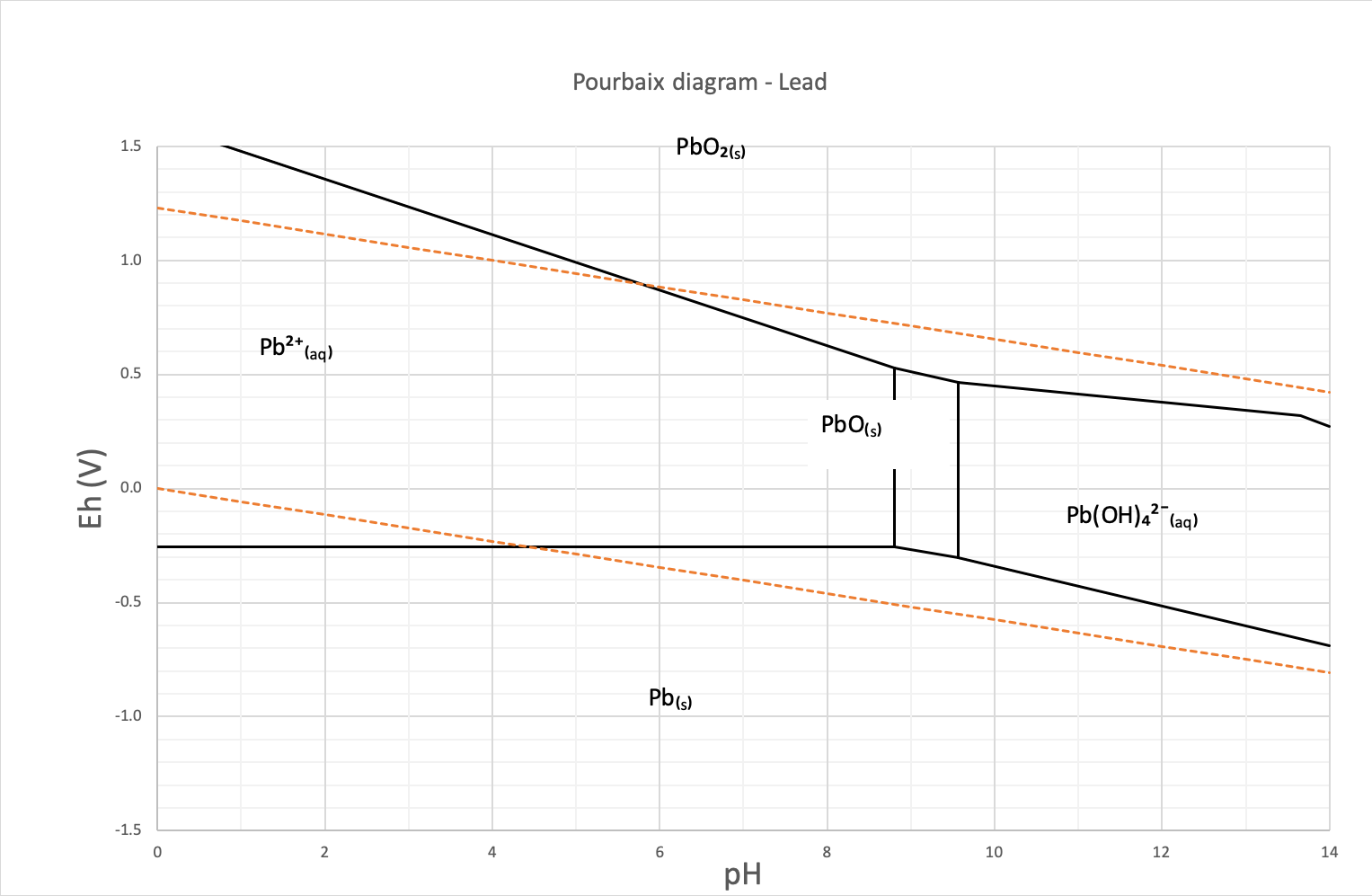
The lead concentration in natural water is generally below 5 ppb (µg/l), however, a contaminated area may contain groundwater with concentration exceeding the World Health Organization (WHO) guideline of 10 ppb (µg/l).
How is lead (Pb²⁺) removed from groundwater
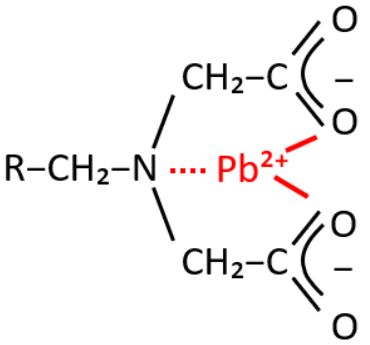
The cationic elementary form of lead (Pb²⁺) can be efficiently captured and removed from groundwater using a weak acid cation (WAC) selective chelating resin with an iminodiacetic group, such as Resinex™ CH-23.
Figure 2: A selective chelating resin with an iminodiacetic group
The average cationic lead (Pb²⁺) removal capacity of Resinex™ CH-23 is 90g of Pb²⁺ per litre resin but depends on the water matrix. Often the water contains other metals which will contribute to the bed exhaustion; therefore, it is always recommended that you contact our technical experts for sizing of a heavy metal removal system as other metals can influence the estimated consumption.
Resinex™ CH-23 is a chelating resin that also efficiently removes other heavy metal cations, such as copper (Cu²⁺), vanadium (Va²⁺), nickel (Ni²⁺), zinc (Zn²⁺) and cadmium (Cd²⁺) in their elementary cationic form.
Recommended lead (Pb²⁺) removal operating conditions
The selection of the operating conditions of a lead removal system depends on:
- Minimum superficial contact time necessary of the application
- Other cationic species in the water
- Desired bed life
- Operating conditions (continuous, discontinuous)
One AquaFlow™ can be used for the removal of lead (Pb²⁺) at a superficial contact time of a minimum of six minutes but usually two AquaFlow™ in series, merry-go-round systems are used with a typical flow rate per vessel: 10- 20 BV/h.
A calculated Lead (Pb²⁺) removal case
Assume a contaminated groundwater with 1 mg/l lead (Pb²⁺) that needs to be purified with the following properties:
- Average flow of 30m³/h
- Continuous operating 24/24 -7/7
The annual lead (Pb²⁺) load is then calculated as follows:
- Nickel concentration: 1mg/l = 1g/m³
- Annual with lead (Pb²⁺) contaminated ground water volume: 30m³/h x 8,600hpa= 258,000m³
- The annual lead (Pb²⁺) load: 1g/m³ x 258,000m³= 258,000g = 258kg Pb²⁺
This gives at an average loading of 90kg Pb²⁺/m³ Resinex™ CH-23 a consumption of approximately 3m³ per annum. An AquaFlow™ filter can be selected from our industrial mobile filter range in order to obtain the desired exchange frequency.
It is always recommended that you contact our technical expert for sizing of a heavy metal removal system as other metals can interfere in the estimated consumption.



1 Comment