Where does chromium come from?
Chromium, which is evenly distributed in the earth’s crust, is obtained from chromite ores, an iron chromium oxide mineral (FeCr₂O₄). The hexavalent chromium (Cr˅ˡ⁺) is very toxic and has both mutagenic and carcinogenic effects, in contrast to the trivalent elementary chromium (Crˡˡˡ⁺), which is an essential nutrient.
Figure 1: Chromite ore.

Chromium has been widely used for many years in various industrial processes as a corrosion inhibitor, pigment and colour fixing agent. (E.g., in cooling towers, electroplating and in the textile industry). The use of chromium-based compounds practice tends to decrease drastically because of health and environmental concern. Groundwater of contaminated areas may still exhibit concentrations above the World Health Organization (WHO) guideline of 50 µg/l as total chromium.
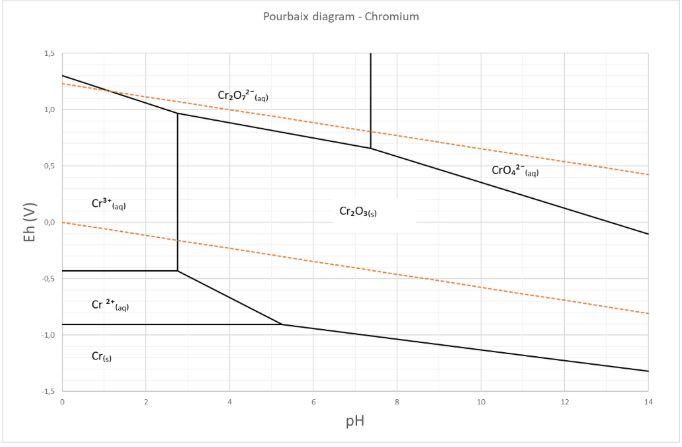
The Pourbaix or potential/pH diagram in Figure 2 of chromium shows the chromium speciation in water. The area between the orange dotted lines represents the stability zone of water. Figure 1 shows that the chromium speciation in water is dominated by chromate (CrO₄²¯), dichromates (Cr₂O₇²¯) and the trivalent elementary chromium (Cr³⁺). Chromate (CrO₄²¯) and dichromate (Cr₂O₇²¯) are oxyanions of the hexavalent chromate (Cr˅ˡ⁺).
Figure 2: The Pourbaix or potential/pH diagram of chromium species.
How is chromium removed from water?
When chromium is present as anionic complexes like chromate (CrO₄²-) or dichromate (Cr₂O₇²¯), a strong base anion (SBA) resin, such as Resinex™ A-4, can be used with a comparable efficiency as a chelating resin. The quaternary-amino group of Resinex™ A-4 exhibits a very high affinity for chromate and dichromate ions. The resins cannot be regenerated. The removal mechanism is described by the reaction below:
2 R-N+(CH₃)₃ Cl- + CrO₄²¯ → 2 [R-N+(CH₃)₃] CrO₄²- + 2 Cl¯
2 R-N+(CH₃)₃ Cl- + Cr₂O₇²¯ → 2 [R-N+(CH₃)₃] Cr₂O₇²+ 2 Cl¯
With an inlet concentration below 200mg/l, the operating capacity of Resinex™ A-4 is expected to be +/- 12 g expressed as Cr per litre of resin (+/-12 kg/m³).
Recommended chromium removal operating conditions
One AquaFlow™ can be used for the removal of chromium at a superficial contact time of a minimum of six minutes, but usually two AquaFlow™ in series, merry-go-round systems are used with a typical flow rate per vessel of 10-30 bed volumes per hour (BV/h).
How to design a chromium removal system?
Assume a contaminated groundwater with 0.5 mg/l as Cr of chromate (Cr˅ˡ⁺) that needs to be purified with following properties:
- Average flow of 30m³/h
- Continuous operating 24/24 -7/7
- Chromium concentrations of 0.5mg/l = 0.5g/m³
- Annual with chromium contaminated groundwater 30m³/h x 8600hpa = 258,000m³
- The annual chromium load: 0.5g/m³ x 258,000m³ = 129,000g = 129kg chromium
This gives at an average loading of 12 kg Cr/m³ Resinex™ A-4 a consumption of approximately 11m³ Resinex™ A-4 per annum. An AquaFlow™ can then be selected from our industrial mobile filter range in order to obtain the desired exchange frequency.
We always recommend you to contact our technical experts for sizing of a heavy metal removal system as other metals can interfere in the estimated consumption.



1 Comment