Introduction
Mercury species exists in various forms: elemental (or metallic); inorganic (e.g. mercuric chloride); and organic mercury (e.g., methyl- and ethylmercury), which all have different toxic effects. Mercury poisoning can result from exposure to water-soluble forms of mercury (such as mercuric chloride or methylmercury), inhalation of mercury vapour, or by ingesting any form of mercury. Mercury contaminated sites can often be found at historical production sites such as:- Fluorescent lamp manufacturing sites
- Fluorescent lamp and battery recycling facilities
- Mercury battery production plants
- Mercury mining sites
- Old alkali chloride-electrolyses according to the mercury cell process
- Plants for fossil fuel and waste combustion
- Production of mercury-containing chemicals and fungicides
- Thermal decontamination facilities of soils containing mercury
- Type of mercury species
- Mercury carrier
- Mercury removal from air
- Mercury removal from water
What are the mercury species in groundwater?
Figure 1 illustrated the simplified Pourbaix diagram of mercury in water. The area between the dotted orange lines represents the stability zone of water. The simplified Pourbaix diagram shows that mercury is mainly found in its solid elementary form Hg⁰ in contaminated water. However, traces of soluble mineral Hg²⁺ are also present and often exceed the World Health Organization (WHO) guideline value of 6 ppb.
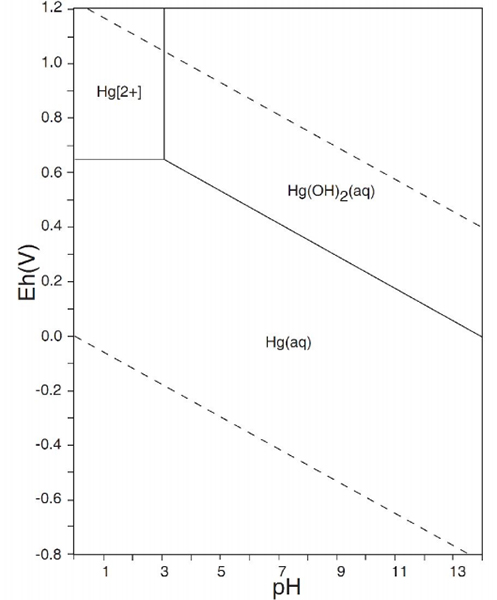
Figure 1: The simplified Pourbaix diagram of mercury in water.
Removal of elementary (metallic) mercury (Hg⁰) from water using activated carbon
AddSorb™ VQ1 is an impregnated grade of activated carbon which uses a chemisorption process for the removal of mercury (Hg⁰) in the aqueous phase. This grade bound the mercury chemical on with the impregnate on the internal surface of the carbon for safe disposal of the spent carbon. Loadings in excess of 60% w/w are possible with this material. Available as a granular and pelletised form, AddSorb™ VQ1 can be supplied to meet all pressure loss requirements. In addition, AddSorb™ VQ1 is also suitable for mercury removal from air.
Removal of ionic mercury (Hg²⁺) from water using resins
Traces of Hg²⁺ can be efficiently captured and removed from groundwater using a weak acid cation (WAC) selective chelating resin such as Resinex™ CH-80. Typical capacity is +/- 20 g as Hg²⁺ per litre of Resinex™ CH-80 resin.
Recommended Mercury (Hg²⁺) removal operating conditions
One AquaFlow™ can be used for the removal of Mercury (Hg²⁺) at a superficial contact time of a minimum of six minutes but usually two AquaFlow™ in series, merry-go-round system are used with a typical flow rate per vessel of 10- 20 bed volumes per hour (BV/h). An upstream sodium hydrosulphite (Na₂S₂O₄) dosage to maintain inlet redox potential below 0.15 V is recommended.
How to design a system to remove Mercury (Hg²⁺) from groundwater
Assume a contaminated groundwater with 0,05 mg/l mercury as (Hg²⁺) and 0.002 mEq/L of Ni and Pb with an average flow of 30m³/h operating 24/24 -7/7 then we have 0,05 g/ m³ x 30m³/h x 8600 hpa (1000 g/kg) = 13kg Mercury (Hg²⁺) per year. This gives at an average loading of 20kg Hg²⁺/m³ Resinex™ CH-80 a consumption of approximately 1m³ Resinex™ CH-80 per annum. An AquaFlow™ can then be selected from our industrial mobile filter range in order to obtain the desired exchange frequency.
Often the water contains other metals which will contribute to the bed exhaustion; therefore, it is always recommended that you contact our technical experts for sizing of a heavy metal removal system as other metals can interfere in the estimated consumption.


